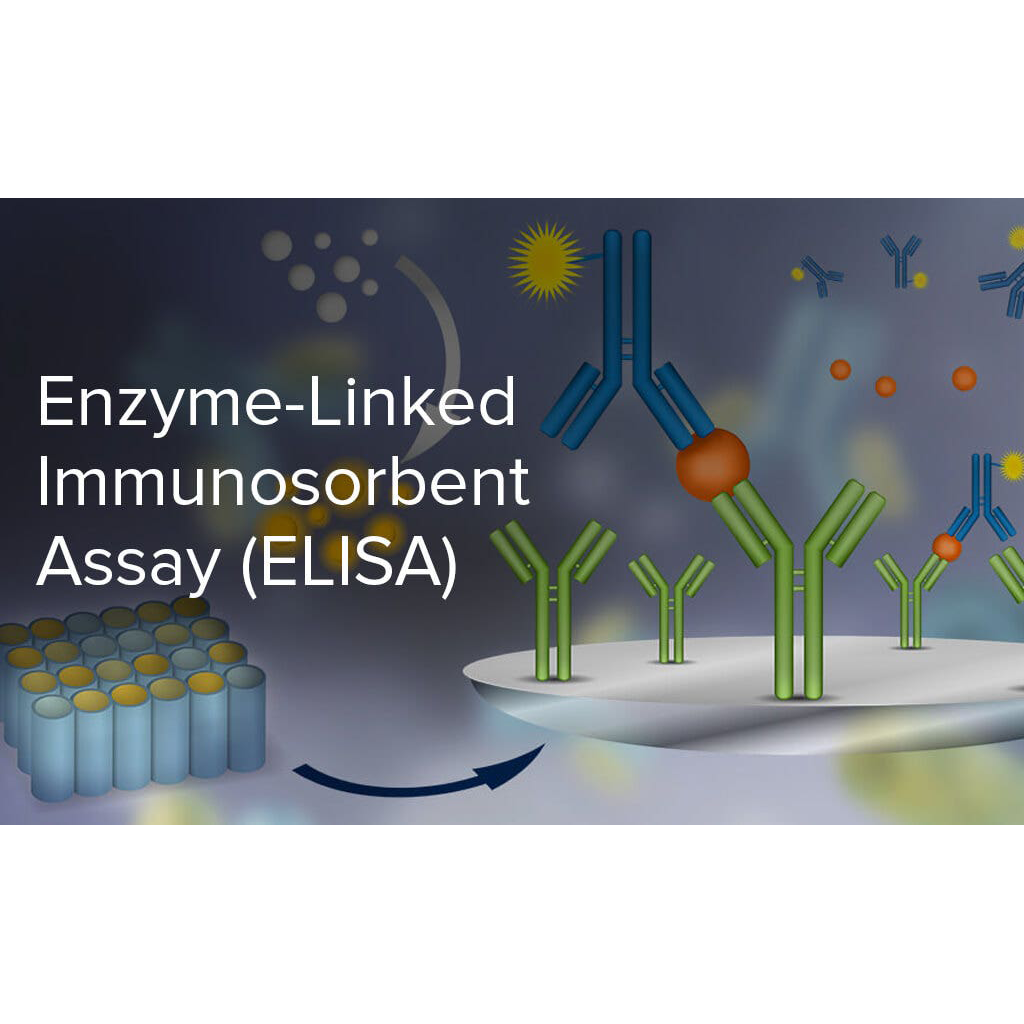
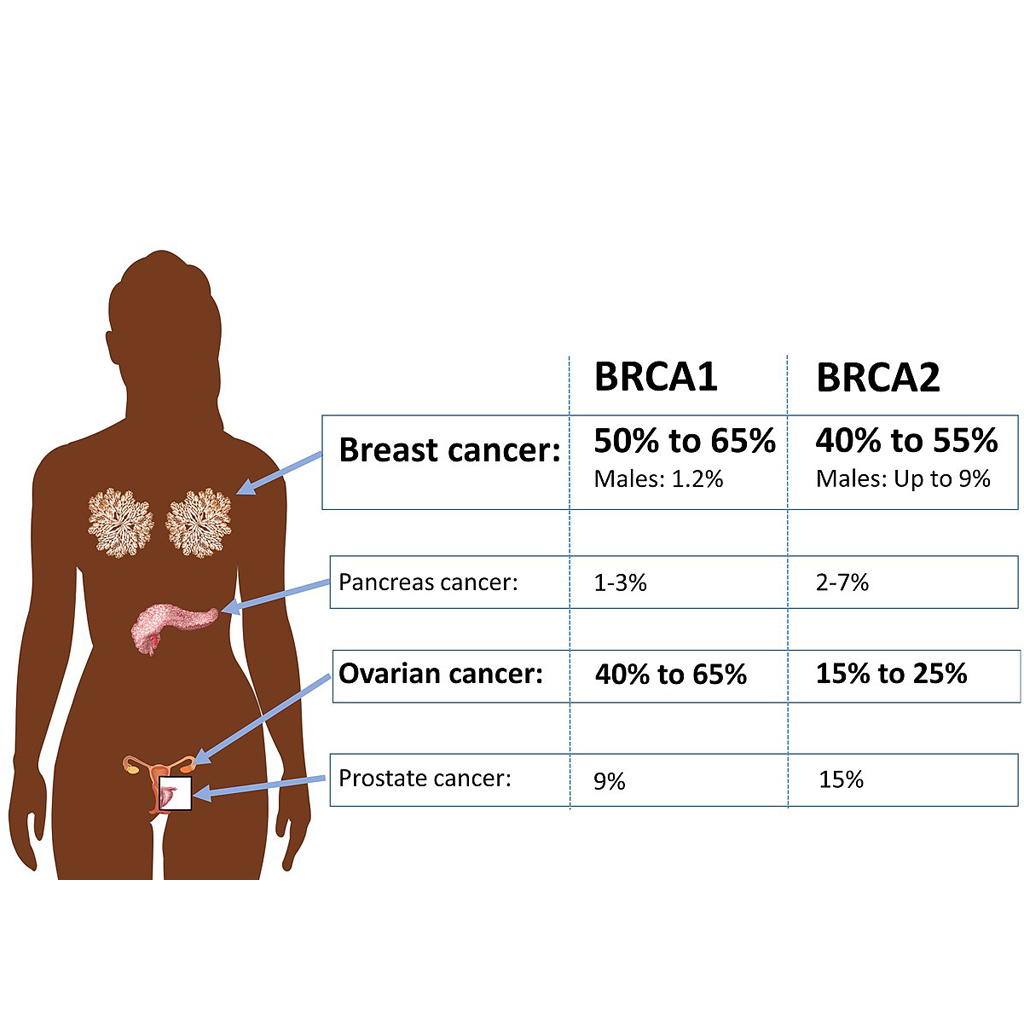
- Explain the primary variables that impact the speed of a chemical reaction (concentration of reactants, temperature, presence of a solvent, utilization of a catalyst), and provide instances of their influence.
- Evaluate the rate of reaction in straightforward chemical processes by analyzing concentration data over time.
- Clarify the connection between temperature and reaction rate.
- Discuss the association between collision theory and activation energy.
- Analyze reaction energy diagrams and link them to energy alterations throughout a reaction.
- Propose an appropriate experimental arrangement for gauging the kinetics of a reaction.
imaginX is used by many amazing schools and universities
University / College