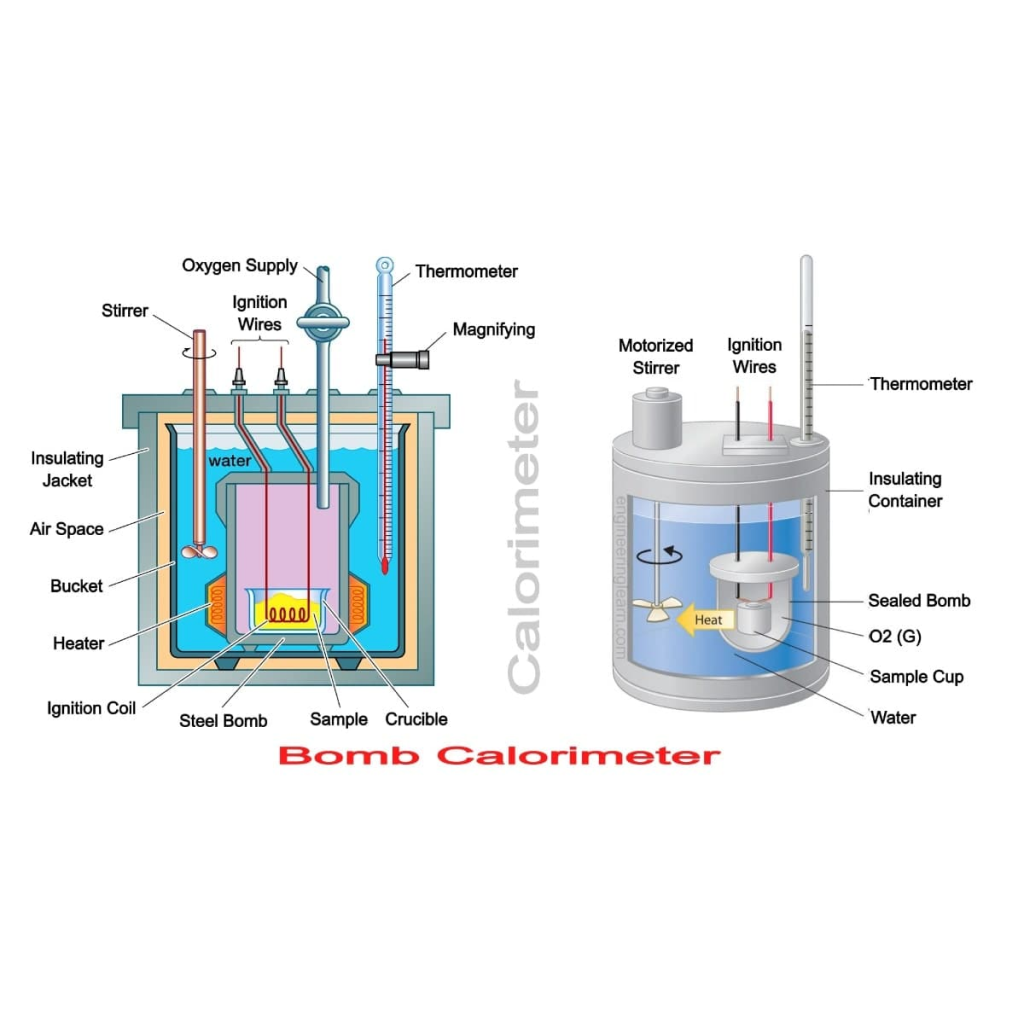
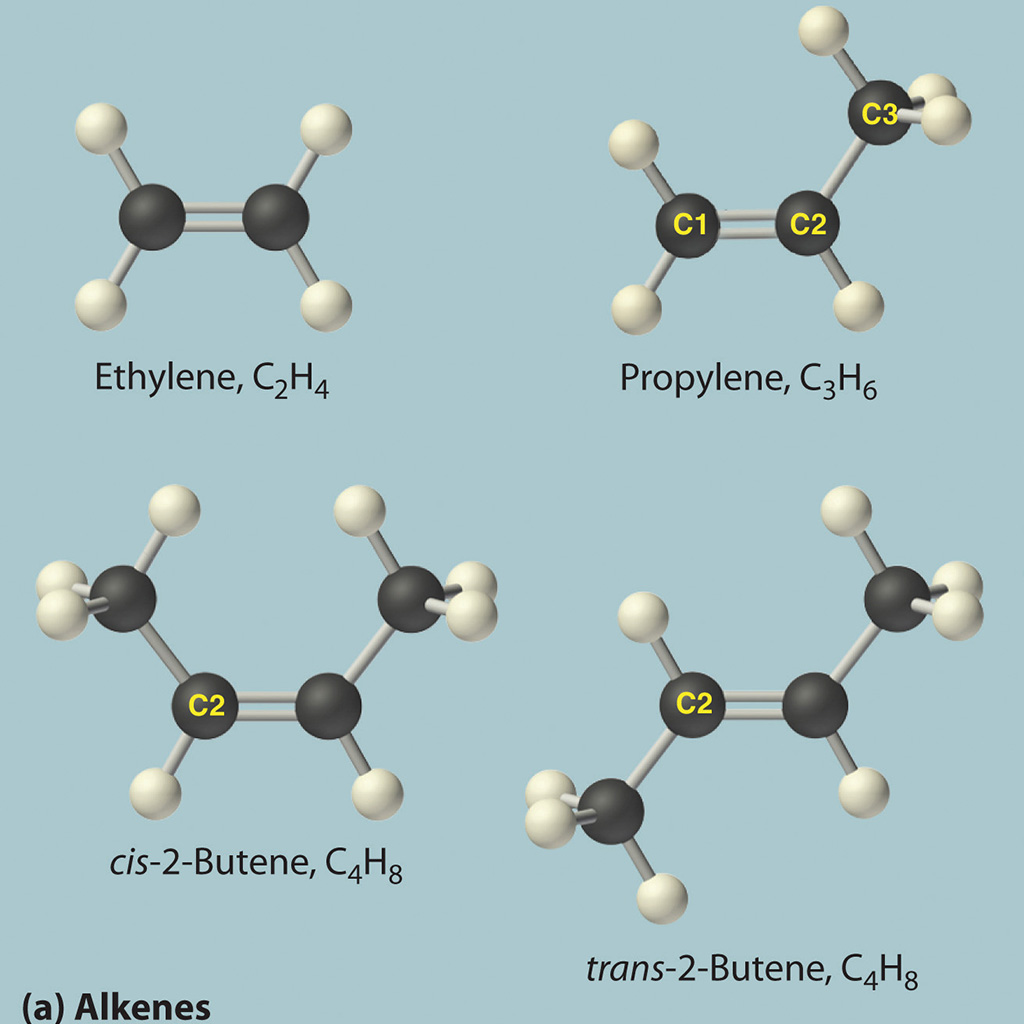
- Offer precise definitions for the fundamental thermodynamic concepts of entropy, enthalpy, and Gibbs free energy.
- Provide a comprehensive explanation of the second law of thermodynamics.
- Grasp and apply the notion of spontaneity in chemical reactions.
- Elaborate on the distinctions between the enthalpy of combustion and the enthalpy of formation.
- Introduce Hesss law in the context of conducting calculations involving changes in enthalpy.
- Discuss the principles behind exothermic and endothermic reactions.
imaginX is used by many amazing schools and universities
University / College